by Oliver Esman
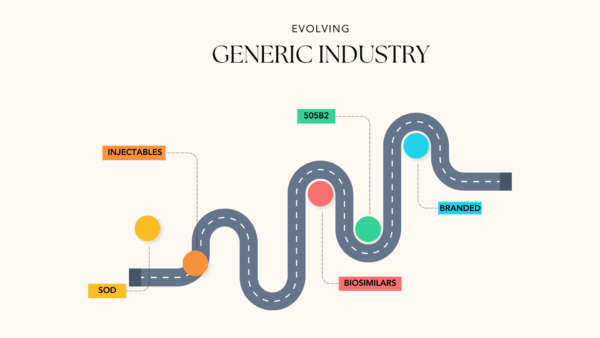
Over the past 20 years there has been a remarkable transformation of the generic industry space as the industry has moved away from dependence on commodity solid oral dose (SOD) generics into higher margin products. My search practice has followed the industry. When I began 18 years ago, the bulk of the industry was still focused on SOD products. As the patents fell on attractive and more complex injectables and solid orals, the industry began a shift to more complex products. Many companies offshored commodity products to less expensive manufacturing geographies, specifically India leading in part to the rapid growth of the Indian pharmaceutical manufacturing sector.
More recently many generics companies have begun to move into 505b2 type products, biosimilars, and branded products of all stripes, pursuing higher margins and longer patent protection. As that process continues, generic companies are more properly becoming specialty pharmaceutical companies with broader and more profitable product lines that often include complex generics, biosimilars, and branded products.
This process has also required companies to look at a broader range of talent throughout their companies to meet the demands of developing and marketing this broad range of products. As the industry has changed our search practice has followed. We now regularly recruit for branded, biosimilars, and complex 505b2 experience in a range of critical functions from commercial activities to manufacturing, quality technical, regulatory affairs, and R&D.
There continues to be strong demand for talent throughout our evolving industry. We expect that to continue as we weather the current choppy business climate. Supply chain instability, payer pressure, regulatory headwinds, pricing reforms, and competition, among other market forces, will only increase the demand for top executives who can lead in this environment. It has been an exciting journey to both watch the development of the industry and be an active participant.

